All for One and One for All
Interoperable Ecosystem for DSCSA
The Interoperable Ecosystem
The industry will need the ability to collaborate with each other and connect to relevant distributed data sets within an interoperable ecosystem by November 2023.
The method of communication and data transmission will utilize the internet. To do so, each dataset will need to have a URL address that fields the acceptance of a request and can facilitate a response. The URL addresses will need to be known and shared among credentialed stakeholders. These are a given.
Two viable interoperable models have emerged as evidenced by the GS1 Lightweight Messaging Standard for the Verification of Product which codified both.
- B2B exchange between data sets with a Lookup Directory to facilitate lookup of the URL
- B2B exchange between data sets with a GS1 conformant Digital link Resolver maintaining a URL to facilitate connectivity
Scalability
Are both approaches (1&2) scalable? Yes.
The Lookup Directory approach scales when multiple directories are created and dedicated to a specific type of context. For example, a VRS Lookup directory currently exists for product verification. Based on recent PDG Verification Architectures discussion, this context type needs to be expanded into PI Verification subtypes. Specifically:
1. Saleable Return (exists)
2. Suspect or Illegitimate
3. Exception Processing
4. Status Check
The VRS Lookup Directory today is made up of 9 separate directories and I am aware of a 10th directory that is to be added. These directories are dedicated to Saleable Returns that are required to be “synced” with each other to remain updated. With our current understanding of product verification types, we now have in the Lookup Directory model the need for 36 separate directories that, by agreement, remain in synchronization with each other.
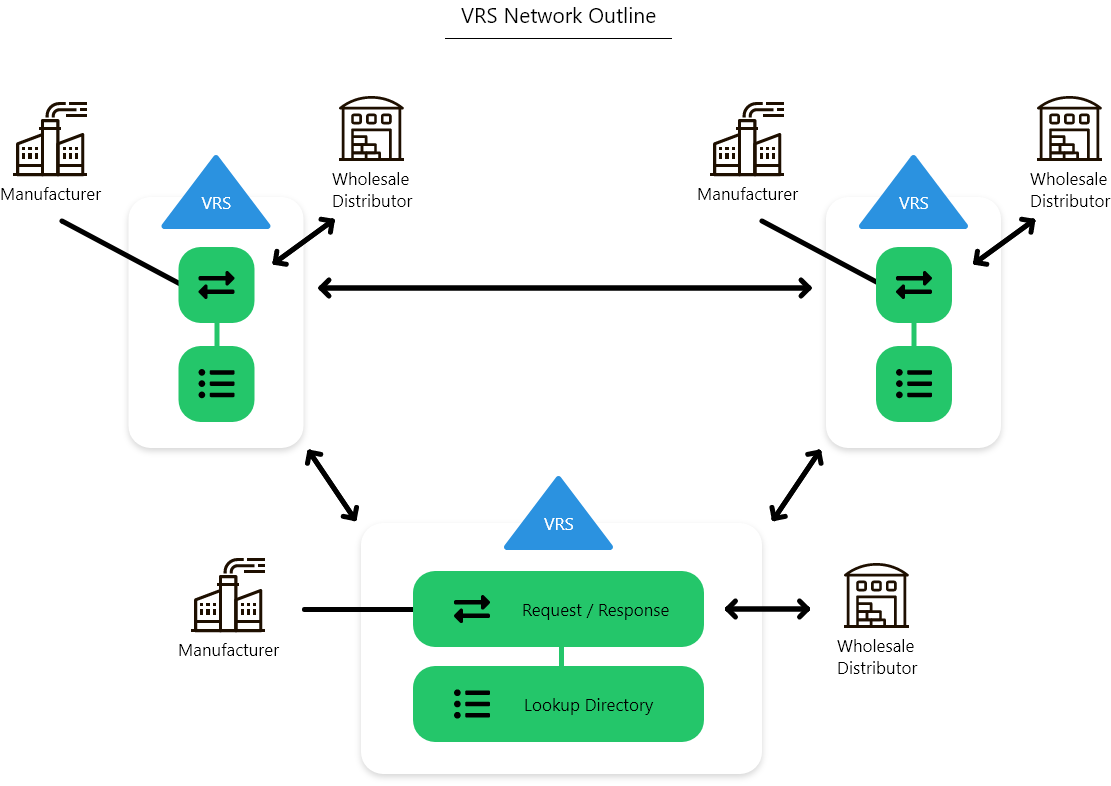
In addition, a directory would also need to be established for other “context types” for the purpose of Trace Request, Recall, and any other types that are determined. This approach is scalable but the ability to achieve and retain synchronization is extremely challenging and may not be sustainable as it relies on multiple parties to assure it syncs. Alternatively, the resolver model only relies on a single entity who is authoritative over their data set to establish and maintain the URL endpoint.
True "Scalability"
The Resolver approach scales when a new context type is added to the resolver. The GS1 Digital Link Standard refers to these context types as “Link Types”. In this model each authority pertaining to a product (NDC/GTIN) or an entity (GLN/DID/DUNS, etc.) establishes a URL address for each needed Link Type adding it to the list of links in their chosen resolver.
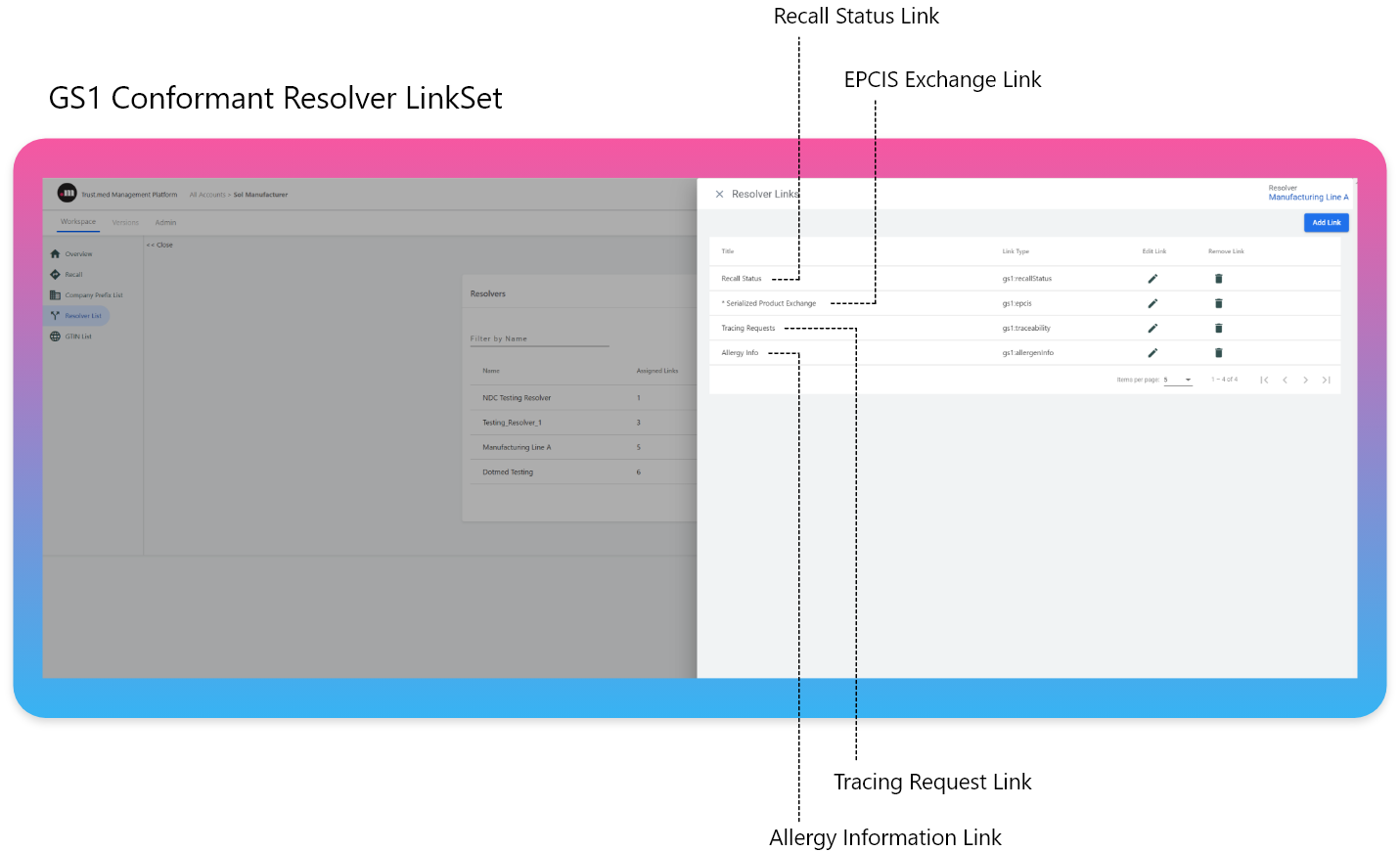
This model scales appropriately and provides a method of auditable control for each actor in the ecosystem to communicate their authoritative endpoint (URL address) for collaborative access to representative systems of record.
This is the discussion that the industry needs to start having today if we are to establish a framework for a secure enhanced drug distribution ecosystem. Both models outlined above are now established in the US. We have established a customer base who will expose their endpoints through a .med GS1 conformant Digital Link resolver and will remain interoperable with any lookup directory that emerges. I am hopeful that PDG facilitates a discussion on these two models as they are both viable and in practice.





