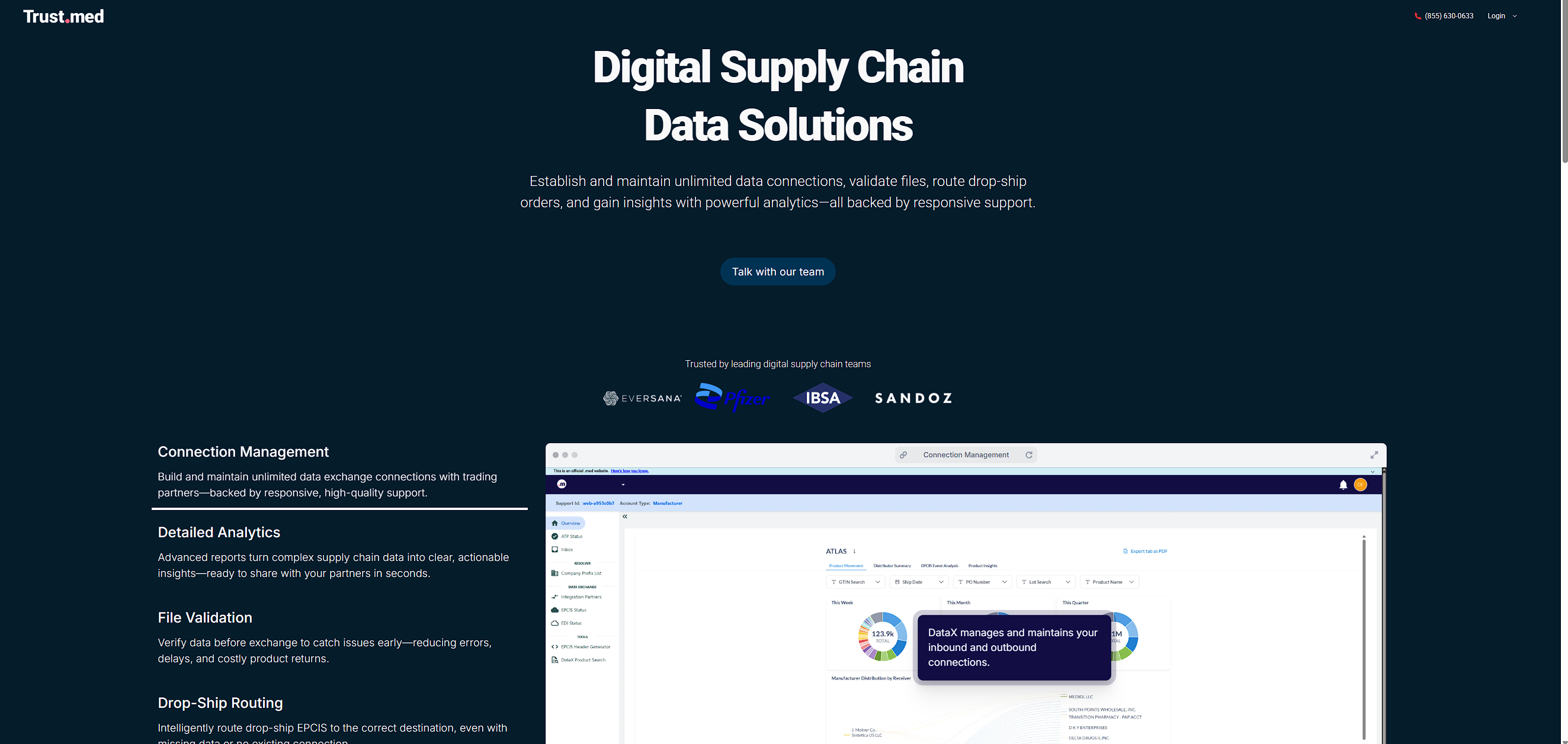
Digital Supply Chain
Data Solutions
Establish and maintain unlimited data connections, validate files, route drop-ship orders, and gain insights with powerful analytics. All backed by responsive support.
Trusted by leading digital supply chain teams




Exchange with DataX
Partner
Compatibility
100%
Production
Endpoints
Unlimited
Simplify data exchange, boost efficiency, and onboard new partners—on a platform with unlimited, fully managed connections and powerful features ready to use.

Share serialized product data with your trading network.
Visualize, query, and understand serialized data in real time.
Automatically route and tag drop-ship EPCIS files.
EDI
Route Advanced Shipping Notices and more.
Validate EPCIS files on the fly with built-in rule checks.
Transformation
Convert your data to the format your partners need.

Engineered for Compliance.
Built for Scale.
Keeping up with evolving regulations and diverse trading partner demands is no easy task. Disparate systems, shifting formats, and missing connections can slow down even the most well-run supply chains.
DataX removes the complexity—standardizing data exchange across your network so you can move faster, stay compliant, and onboard new partners without operational overhead.
"In the fragmented medical supply chain market, transparency remains an opportunity. Trust.med offers an ecosystem used to track and verify the provenance of medicine by bringing transparency to the supply chain through secure protocols that simplify the exchange of this data. In practice, this means sharing more information between parties like health care manufacturers, consumers, health care and technology providers, distributors, and regulatory agencies, making collaboration much easier, effective, and aligned with everyone's needs."
"For those frustrated by technicalities, unprepared contractors or other obstacles to setting up systems, it’s not too late to outsource. An outsourced solution can remove the system administration burden and the cost of data storage and even manage connections and communications with trade partners."
"Trust.med ensures secure, uninterrupted access to critical pharmaceutical product information, enabling seamless data exchange, recall notifications, and product verification. More than just a technological tool, Trust.med aids manufacturers in meeting the stringent DSCSA compliance requirements, enhancing the global discoverability of pharmaceutical product and location data."
“From a data exchange perspective... we earn business from manufacturers that are frustrated with the controls that their solution providers give them today,”
"A new approach to both trading-partner verification and to establishing EPCIS-compliant messaging...
In effect, Trust.med uses the machinery of the internet to manage the security and the interoperability of DSCSA compliance."