Registry Launch
Today we launched the Trust.med Registry
The registry is a catalog of drug product identifiers, paired with manufacturer contact information, developed to enhance communication on the drug supply chain. It’s searchable by National Drug Code (NDC) or Global Trade Item Number (GTIN).

The Drug Supply Chain Security Act (DSCSA) highlighted communications barriers. These barriers are impeding some trading partners from complying with regulations. This led to the launch of our registry, which allows organizations along the supply chain to identify the appropriate contact information for critical communications like those required by the DSCSA. In the near future, it will facilitate partner-to-partner messaging, list product statuses, allow partners to exchange master data, and more.
In this blog, I’ll walk you through what is included in the registry at launch, discuss its mission of enhancing supply chain communication, and touch on some of the updates coming to the registry.
How It Works
The DSCSA regulation introduced requirements for trading partners to communicate in new ways. The ability to find contact information and establish communication is a priority.
A registry of contact details needs to be:
- easy to access for all trading partners regardless of technical capabilities
- searchable by product ID to account for scenarios where the manufacturer’s name is unknown
At launch there are over 40,000 searchable drug products in the Trust.med Registry.
Using the NDC as the primary ID for product listings within the registry made the most sense. Each product listing is paired with the manufacturer's associated contact information.
The NDC is identifiable on the product label and is an ID that regulators can rely on. This is why we chose it as the primary ID for searching product listings. The GTIN has a tremendous amount of value as well, especially when the searcher is looking for more granular information. Product listings can also be searched by GTIN.
As the operator of the .med Top Level Domain (TLD), we are an authoritative registry on the internet of domain name registrations and related contact information. Much like .gov or .edu, verification of identity is required. Indirect trading partners in particular are able to easily establish connection at scale to meet pressing DSCSA needs to securely exchange information.
A user is able to search the registry by NDC or GTIN to locate the manufacturer of record. If a matching drug product exists in the registry, the user is taken to a product listing where information about the product is displayed along with associated contact details of the manufacturer.
At launch there are over 40,000 searchable drug products in the Trust.med Registry.
You can search the registry here: Trust.med
The registry is designed for interoperability from the ground up. Products not yet contained in the registry can be added at no charge.
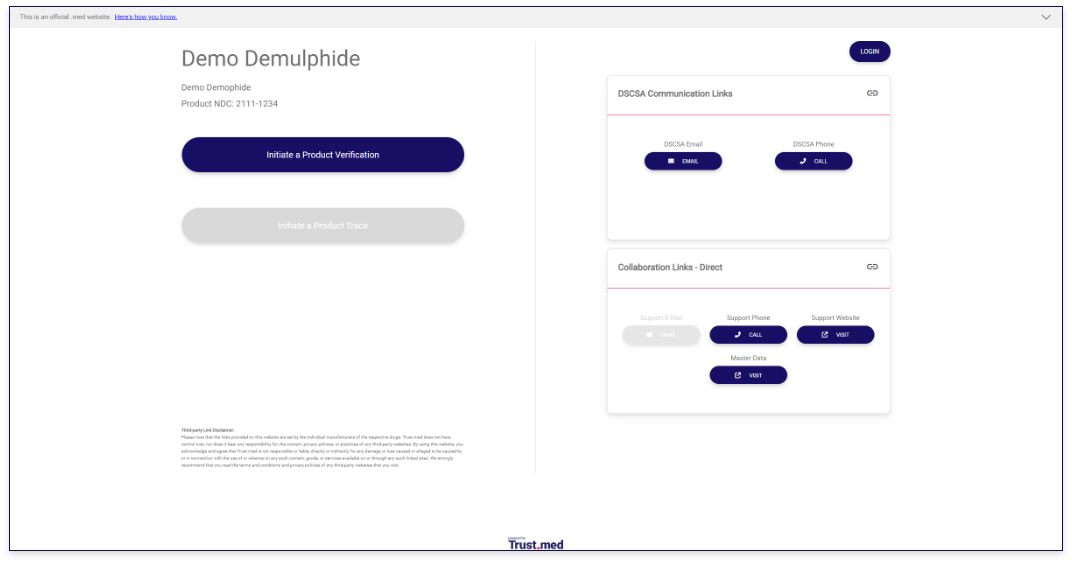
Each product listing in the registry uses a GS1 conformant resolver to display product and contact information. This uniformly gives owners of information, and those seeking information, a standard approach to editing, displaying, and interacting with the product listings.
GS1’s Digital Link and Lightweight Messaging Standards are also used when communicating with services through the registry. The registry will soon give users the ability to create and send partner-to-partner messages. These standards are used for requests across the registry, making integration to the service a negligible task.
Trust.med is a certified GS1 solution provider.
Editing Product Listings
In order to add or edit product listings and contact details within the registry, you must validate ownership of the drug product listed.
Manufacturers can use Trust.med’s portal to then manage the contact details that are shown on their product listings. By default each product listing shows the following DSCSA specific details:
- DSCSA Phone - the phone number where you prefer to receive DSCSA related calls
- DSCSA Email - the email address where you prefer to receive DSCSA related emails
- (optional) DSCSA Website - the website where you want to send users who want DSCSA related information
Manufacturers can also manage other details specific to their products from within their user-friendly portal.
There are many ways to utilize the registry for enhanced communications. I look forward to highlighting more ways to use the registry in our first post-launch update coming in November of this year.
To update the contact information on your product listings fill out this form:
https://trust.med/registration/
A member of our team will follow up with you to guide you through the 10 minute product ownership validation process.
Accessing the Registry
The registry is publicly accessible to any user with internet access.
DSCSA related actions require user authorization via a straight forward sign-up process.
The 3 primary ways of accessing the registry are:
Trust.med Search
A search bar is available on Trust.med. It allows users to search the registry by manually entering an NDC or GTIN. Users can also select the scan button to enable the Trust.med 2D Data Matrix Scanner. This uses the camera on your phone or computer and will allow you to scan a 2D matrix to locate a product listing.
If a product listing matches your search entry you will be taken to the product listing in a new browser tab.
Address Bar in Browser
The address bar in any web browser can be used to locate product information. Since each product listing is hosted as a URL, a user can type the NDC or GTIN followed by ‘.med’ into their browser's address bar for direct navigation to the product listing.
Here is an example NDC search in the URL bar:

Integration
The registry can be integrated into other services and applications. The registry and its full capabilities are available via API (Application Programming Interface). Developers can utilize the registry to add additional functionality and benefits to their existing products. This gives trading partners the ability to utilize their existing solution providers and seamlessly integrate enhanced supply chain communications into their operating procedures.
Pricing
- Listing products by NDC in the registry is free.
- Searching the registry is also free.
Elevated features like notification of FDA approved product exemptions / waiver status, enhanced recall, and exception handling notifications carry a minimal product based fee.
The Future of the Registry
On the horizon are two significant updates to the registry.
The first update will come in the form of including the registry in Trust.med’s Connect API. This will allow solution providers to search product listings and gather contact information from within their own applications, enhancing their customers' ability to collaborate.
The second update will pair the registry with our request and broadcast services. This will give users and partners the ability to perform product verifications, exchange data, set product exemption flags, and more.
Meeting DSCSA requirements remains a challenge for many trading partners. The launch of our registry gives trading partners, regulators, and technology providers a resource to connect and enhance collaboration efforts and meet deadlines.
At launch, our registry promotes the discoverability of manufacturer contact details.
Soon this same communication resource will facilitate asking granular product verification questions like “is this product exempt?”, and simplify trading partner engagement and collaboration.
We are excited to launch the registry and look forward to growing its listings and enhancing its capabilities.





