Sharing Should Be Easy
Have You Achieved Interoperability for DSCSA Compliance?
Interoperability is Achievable
Interoperability is a characteristic of an ability to work with others. DSCSA requires that ATPs be able to work among themselves to exchange product information as needed for the purpose of transaction, product verification or trace for various suspect/illegitimate product reasons, and for enhancement of industry recall mechanisms.
None of this can be achieved if the industry is not connected. This also means securely connected. At a very high level that should only allow a limited set of participants to securely connect and interoperably collaborate. So let’s start by achieving just those two things: Connectivity & Security
A Familiar Solution Already Exists
Without debate, the internet is “The Network” upon which all the world’s computers are connected for sharing data. These connections are endpoints representing digital locations as URL addresses on the internet. Each endpoint is associated with a legal entity responsible for the connection and content available at that endpoint. For companies selling prescription drugs in the United States, the legal entities associated with these endpoints are defined in the DSCSA (Drug Supply Chain Security Act) as an ATP (Authorized Trading Partner). The DSCSA regulation requires “Enhanced Drug Distribution Security” to go into effect by November 2023.
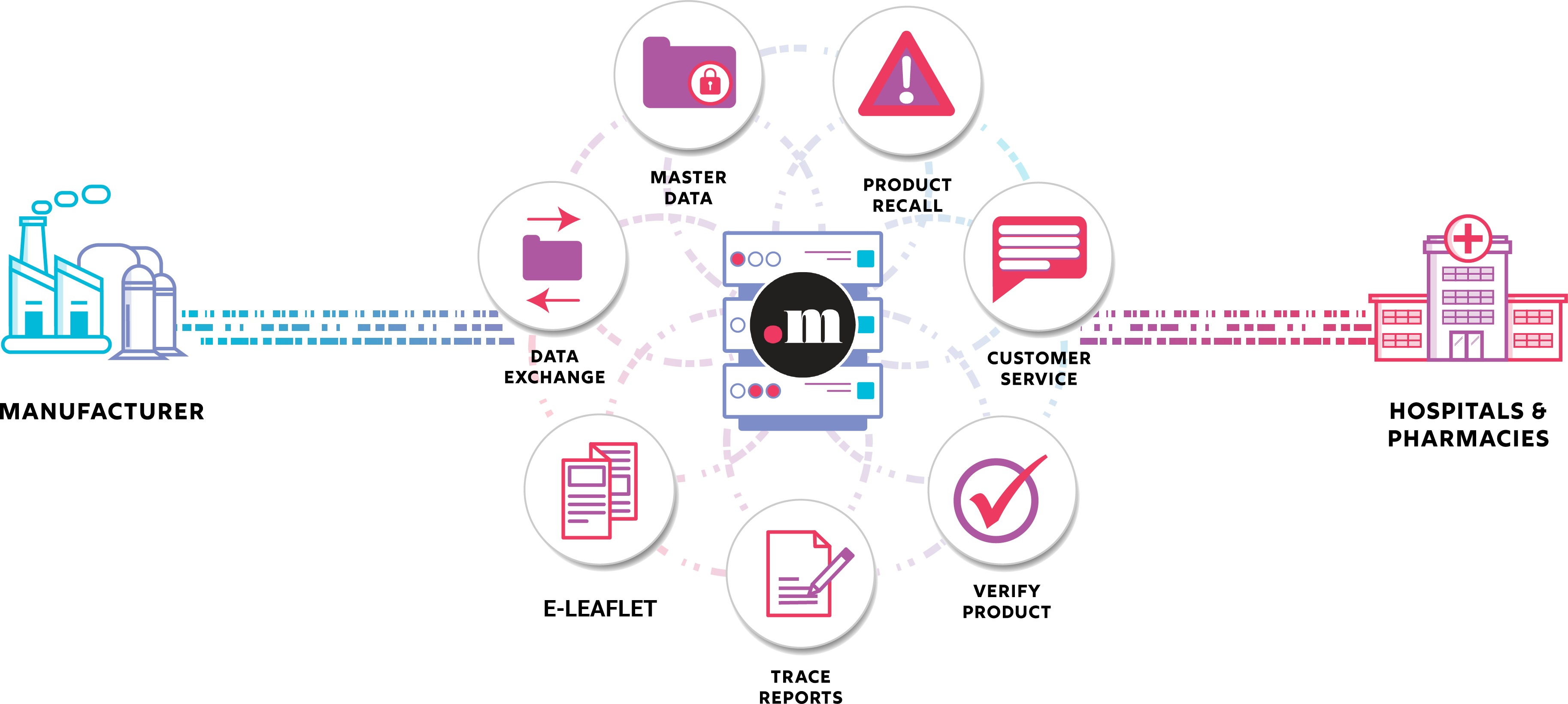
As we approach November 2022 it has become evident that there is no consolidated understanding among the ATPs of what is needed to realize achievement of Enhanced Drug Distribution Security as a unified industry. With a little over a year to go, maybe it’s time to shift the paradigm. This can be done with three simple expectations your organization can embrace and control.
The ATP Challenge: Take Control and Focus on Your Specific Set of Solutions!
1
Does your organization meet DSCSA requirements? Can you prove it?
2
Are your endpoints secure, discoverable, and most importantly interoperable?
3
Will you only interact with others if they can achieve the same expectations?
By approaching the DSCSA interoperability from this perspective, you have the capability to achieve full DSCSA compliance by November 2023. The shift starts with examining your Level 5 capabilities for communication and collaboration with others. Separately examine the L5 function as a standalone set of requirements to gain clarity of your business needs. As part of the process, you will need to understand how your L4 system can interoperate with your L5. To be successful however, Do Not allow a consolidated L4/L5 solution to be the mindset of your approach. Look at your L5 as a separate component of your solution.
I believe that every organization has the capability to achieve full interoperable compliance to meet DSCSA 2023 requirements, if they take a new approach and attack the problem from the simple perspective of management and control of endpoint connections. Please contact me, I welcome any discussion about interoperability and compliance.





