The Drug Manufacturer’s DSCSA Dilemma
10 Years Later, Still Looking for Solutions
A decade's worth of time has expired since the Drug Supply Chain Security Act (DSCSA) was passed to help protect consumers from exposure to drugs that may be counterfeit, stolen, contaminated, or otherwise harmful. This November marks the start of the 12 month countdown requiring pharmaceutical trading partners to comply with the law. As time quickly slips away, trading partners are still left with unanswered questions and serious operational risks if found to be non-compliant by November 27, 2023.
Since the legislation was passed, the pharma supply chain industry has collaborated on several potential interoperable solutions for compliance. However, drug manufacturers are still left searching for solutions for how they will exchange serialized data with their direct consignee dispensers.
This is the “Drug Manufacturer’s DSCSA Dilemma.”
Electronically Exchanging Serialized Product Data
Under the DSCSA, drug manufacturer trading partners are to electronically exchange serialized product data when there is a change in product ownership. This data must be available upon request and stored for a minimum of six years. Trading partners are not able to ship or receive DSCSA regulated products after November 27, 2023 without the shipment's associated serialized product data being exchanged.
On July 6, 2022 the FDA provided an update to its draft guidance regarding the interoperable exchange of information. It states that the “FDA recommends that trading partners use the Electronic Product Code Information Services (EPCIS) standard to provide and maintain the data associated with transaction information and transaction statements.”
Wholesale distributors and manufacturers have publicly stressed the importance of serialized product data exchange. They Advocate for a standardized approach to the new data exchange requirements. Wholesale distributors emphasized that shipments without serialized product data in EPCIS standard will cease to be accepted. EPCIS is the industry adopted GS1 standard for serialized product tracing event data.
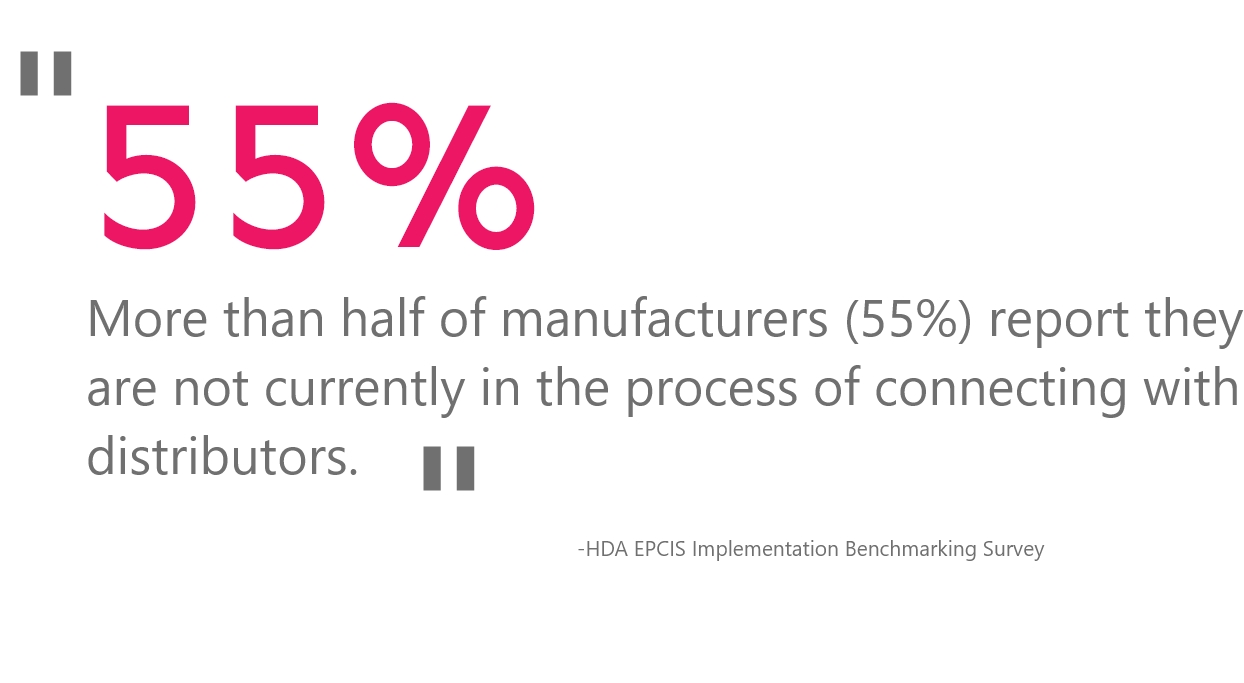
In a recent Healthcare Distribution Alliance (HDA) survey, more than half of manufacturer respondents surveyed (55%) said that they are not in the process of establishing an EPCIS connection to their distributor partners. With roughly 92% of drug products shipping through wholesale distributors, this survey illuminates the sizable amount of work still required so as to not interrupt supply chain operations.
The Dispenser Dilemma
Serialized data exchange, for the lion's share of a manufacturers' products, is a work in progress. However, the exchange of serialized data for the remaining 8% - 10% of their product distribution is a significantly more difficult problem to tackle.
Drug manufacturers’ products that are directly distributed to pharmacies and hospitals carry the same regulatory data exchange requirements as products moved through wholesale distributors. The major difference is that new connections must be established between drug manufacturers and their direct dispenser customers.
In most cases connections between manufacturers and dispensers has been through an Electronic Data Interchange (EDI). This machine-to-machine link, established between trading partners for order-to-cash, is a pipeline unable to optimally carry the serialized data required for compliance.
Up to this point, industry work on the exchange of serialized data has been led by major distributors with a narrow focus on the shipments that travel through their facilities. This has set in motion the standards and integration work required to cover the majority of drugs moved on the pharma supply chain. However, it does not account for the drug manufacturers' direct distribution of products to dispensers. HDA’s most recent readiness benchmark survey highlights that only 13% of drug manufacturers have a plan to connect with their dispenser customers.
A mere 13 months remains (at the time of writing this) for pharmaceutical trading partners to comply with the DSCSA. With no previously established connection, drug manufacturers have minimal time to determine their solutions for exchanging serialization data with their direct dispenser customers.
In just over a year's time all drug manufacturers will need to have established and tested the exchange of serialized data with their wholesale distributors and direct ship dispensers. Failure to do so will result in the inability to move product and result in non-compliance with the federal government's legislation.
EPCIS
Manufacturers tackling the exchange of serialized data must begin their journey by adopting and implementing EPCIS. This first and most basic step is not without its challenges. Specific data repositories, version matching with trade partners, and implementing a system for the testing and evaluation of EPCIS events are fundamental expectations.
It is recommended that EPCIS events meet the GS1 US Rx Standard. Failure to do so jeopardizes exchanged drug supply transactions data. Improperly formatted EPCIS events threaten the delivery of in-progress shipments.
Based on a 2022 survey of Trading Partners top challenges in adopting EPCIS include:
- Trade Partner Understanding / Commitment
- Employee Resources
- IT Resources
Additional obstacles identified by respondents included: system constraints, downstream partner readiness, differing interpretations of DSCSA requirements, and lack of responsiveness from trading partners.
Solution providers, specializing in DSCSA regulated data delivery and trade partner communications, can provide a more granular approach to the control of your data. Giving you the flexibility that comes by separating services between technology providers.
As solution providers are evaluated, ensure that they:
- are ingrained into industry working groups
- have the ability to provide thought leadership
- follow interoperability requirements
- are cost-effective
- give you control of your endpoints and data
Getting Data to the Dispensers
Manufacturers shipping directly to dispensers must set their sights on the delivery of their serialized data to these smaller partners, or run the risk of non-compliance. EPCIS awareness has permeated through the top of the supply chain but many downstream trading partners like specialized pharmacies and hospital systems are looking at their manufacturing and distribution partners to supply them with required DSCSA data.
Drug manufacturers should consider creating EPCIS repositories on behalf of their dispenser partners. EPCIS workspaces / portals allow users to access and manage the product tracing information. Thus, giving dispensers and their manufacturer suppliers control over their data and compliance. Failure to move quickly on planning and implementation of this step could see the exchange of serialized data with dispensers get lost in the chaotic shuffle coming in 2023.
Solving the Dispenser Dilemma
Dispensers will need the ability to view, access, and manage the electronic serialized data that is required to accompany the physical product shipments. Serialized data from manufacturers must be stored and accessible for a minimum of six years after the product has shipped.
“You have 60,000 plus trading partners in the US supply chain and most of them are not sophisticated enough or have the capital or have the infrastructure to build systems to collect this data. They need us (drug manufacturers) to develop portal solutions where they can pull the data,” said Dave Mason of Novartis in a recent article on raps.org.
Options to Consider
Development of a proprietary portal carries significant challenges, time being the most pertinent. As IT resources shift toward the adoption and enablement of EPCIS, the development of a dashboard is a less than ideal option. Features including the management of EPCIS events, user accounts, and customer support functionality make this option a hard pill to swallow.
Level 4 providers may be able to provide services that connect to your dispenser trading partners. High connection fees and development lead time will almost certainly make this a non-starter for manufacturers looking to keep surging technical costs in check while fighting the clock to meet DSCSAs November '23 deadline.
Specialized service providers who focus on Level 5 and the exchange of DSCSA regulated data are the best option. These providers have designed their applications specifically for the purpose of moving serialized data between manufacturers and their downstream partners.
Some technology partners, like Trust.med, have developed infrastructure for the discovery and exchange of regulated information and communications. Utilizing systems, built to solve the manufacturer to dispenser data exchange dilemma, keeps pricing manageable while ticking all of the regulatory boxes. Trust.med provides portals and integrations for dispensers to access and manage EPCIS from their upstream manufacturing partners.
By providing both portals and integrations, manufacturers aren't forced to wait for downstream service providers development resources to become available. After onboarding, EPCIS can immediately be made available to all downstream partners.
Your Next Step
As time expires manufacturers must make their decisions on how they will facilitate the exchange of EPCIS event data. Taking into consideration resources, cost, and time; manufacturers should look for solution providers that offer services to meet this specific need.
The right technology partners will have the ability to:
- onboard dispensers
- provide workspaces / portals to manage tracing data
- interoperate with existing systems
- offer an extensible framework for future needs / growth
The process of determining solution providers takes time and drains limited resources. Starting the selection process now will allow for internal hiccups and inevitable delays to be resolved before November 27, 2023.