EPCIS Data Exchange
EPCIS Data Exchange
Securely Exchange Standardized Data Requests
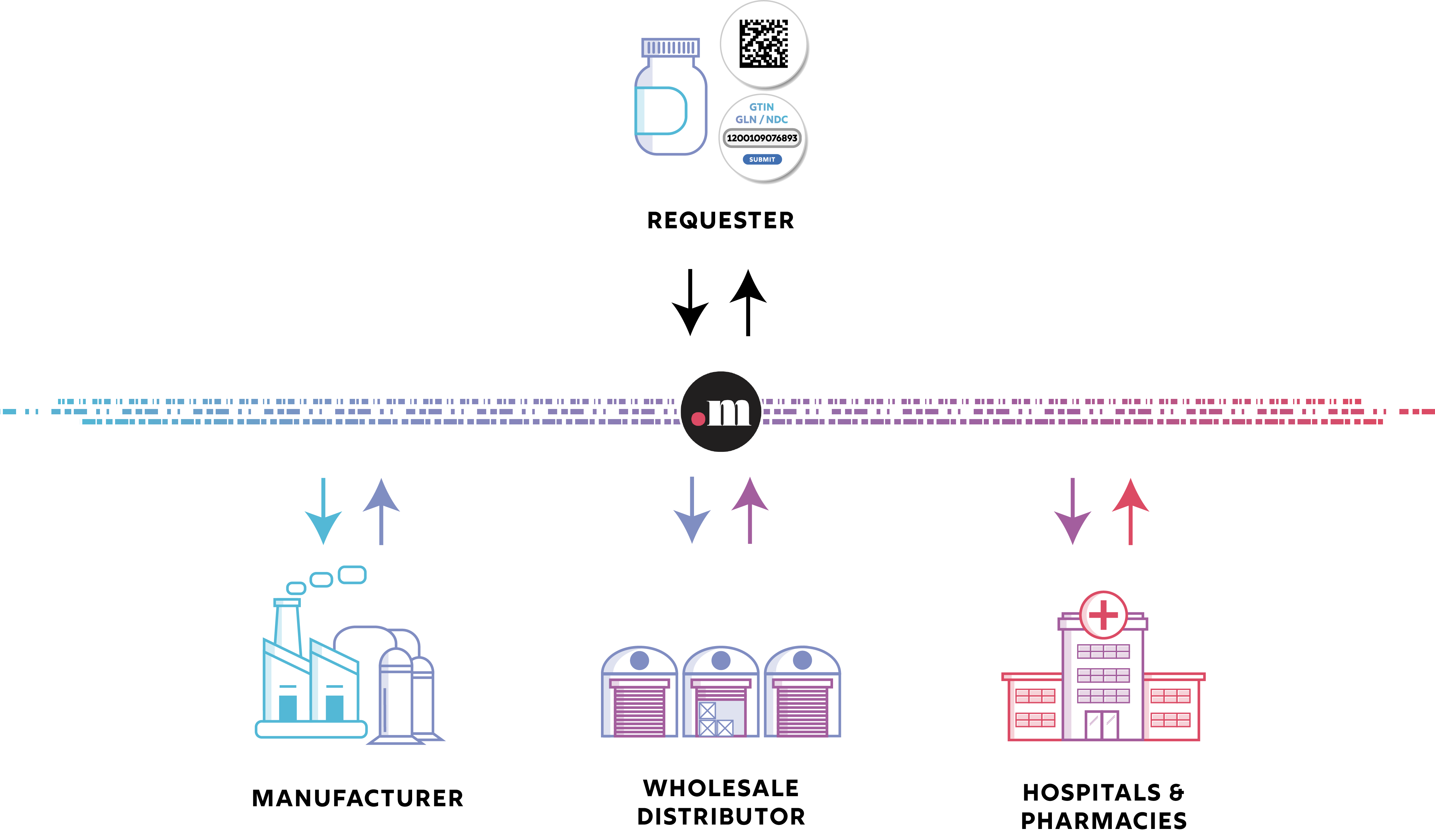
Trust.med’s solution for the electronic exchange of serialized data [DataX] allows trading partners to easily, and securely, exchange serialized drug product data. The ability to provide and access serialized tracing data is a federally mandated requirement under the Drug Supply Chain Security (DSCSA). Serialized data is also referenced as “serialized transaction data,” “serialized T3 data,” “EPCIS,” and “serialized tracing data.”
The Trust.med registry provides secure data exchange and accessibility services for drug manufacturers, wholesale distributors, pharmacies and hospitals. Manufacturers and wholesale distributors can easily map their data endpoints to .med domain names through our Trust.med dashboard or API. Pharmacies and hospitals can also access their serialized tracing data in our dashboard or within their preferred existing in-use application. Trust.med’s solution for the electronic exchange of serialized data is available to all trading partners and drug supply chain solution providers.
Locate Serialized Transaction Data
Discovering the location of data can be a challenge. This is especially true as it relates to DSCSA regulated data. DSCSA requires interactions between companies that are not currently connected.
Trust.med allows trade partner organizations to discover where specific data, like serialized tracing information, is located via our top-level registry.
The Trust.med registry lives at the core of all of Trust.med’s services. Our registry associates products and locations to authorized endpoints where data can be found and accessed. Products are identified within the Trust.med registry using GTINs. Locations are identified using GLNs. Using these GS1 Digital Link standard identifiers ensures requestors have the information necessary to discover the appropriate and authoritative endpoints.
Each GTIN, GLN, or NDC is converted into a "standards based" domain name by Trust.med (e.g.12345678987654.med). This standards based domain name is then assigned a resolver by the trade partner controlling the GS1 identifier. Proof of managing capability for the GS1 identifier is required as GTIN, GLN, or NDC based .med domain names are created within Trust.med. This ensures authoritative trade partner control.
Access restrictions can be set on a per link basis. This ensures data requestors meet authorization requirements, thus preventing irrelevant requests.
Any trade partner can visit a standards based .med domain name and access a list of links, called a link set. These link sets represent where a specific type of data, like serialized tracing information, is located. Our software then connects authorized users to authoritative data endpoints through a resolver. All Trust.med resolvers meet GS1 standards.
Changing the ownership of GTINs, GLNs, or NDCs between entities via mergers, acquisitions, or divestitures are accounted for within the Trust.med registry. Our system accounts for changes in ownership of a product or location as well as changes in service providers that have control of endpoints.
Trust.med’s serialized data exchange for trading partners solves the problem of who to ask for serialized data by implementing and utilizing an internet based registry. This easy method for the discovery of data eliminates the need for point-to-point connections between trading partners.
Request & Access Serialized Transaction Data
After discovering the location of the serialized data, trade partners will then have the ability to query the data set. Querying the data set allows users to retrieve precise product based serialized information all within one platform.
The US Drug Supply Chain comprises thousands of trading partners and service providers. Trust.med’s serialized data exchange uses GS1’s Digital Link to enable any trading partner to make a request to another trading partner. This solves the problem of how to electronically ask for serialized data.
Digital Link is a standard that utilizes ‘application identifiers’, or AI’s, to format a request for information. Trust.med utilizes the AI’s embedded within the 2D Data Matrix on every DSCSA regulated drug product label within its requests for serialized data. Each request made with Trust.med utilizes the following application identifiers:
The Trust.med Digital Link URL pairs industry accepted standards with the .med restricted top-level domain. This unique URL creates a universal syntax for identifying and interacting United States Drug Supply Chain product data.
(01) GTIN - Global Trade Item Number
(10) Batch or Lot Number
(21) Serial Number
(17) Expiration Date
Utilize Industry Adopted Standards
Trust.med utilizes, existing, industry adopted standards for querying serialized data. All of our solutions are interoperable and collaboratively designed with industry experts. Authorized requestors can retrieve serialized product data by:

Authoritative Sources
Access and use of the Trust.med registry is restricted to the Healthcare Supply Chain. Prior to being provided access to the platform users are verified, categorized, and assigned permissions based on role and status. These restrictions ensure that only authoritative entities and users have access to the Trust.med registry.
The .med top-level domain is a restricted top-level domain. Activation of standards based domain names requires proof of authoritative trade partner control over the product or location identifiers. This is accomplished through the submission and review of GS1 identifier license certificates.
Management, Access, and Data Formatting
The serialized data exchange module is accessed from within the Trust.med registry. Access all of Trust.med’s healthcare supply chain software applications in one easy to use solution. Owners of serialized data have the ability to manage product identifiers, domains, resolvers, and links to endpoints. Trading partners who need to retrieve serialized data from upstream trading partners can also access their data through the Trust.med registry.
EPCIS or Electronic Product Code Information Services is the GS1 Standard for creating and sharing visibility event data, both within and across enterprises. All serialized data exchanged by Trust.med is done in EPCIS format. This requires the implementation of EPCIS event capturing by all users.
Trust.med and its network of solution partners can aid your organization in implementing the EPCIS GS1 standard.
Trust.med monitors for adherence to the GS1 EPCIS standard. We flag events that do not meet these specifications ensuring consistency of data exchange and promoting the adoption of this preferred industry format for serialized transaction data.