Aggregration
& Exceptions
Is data aggregation necessary for interoperable trade?
By: Lloyd Mager, Market Solutions Officer
Aggregation and Exceptions
DSCSA does not mandate aggregation. Does that mean you shouldn’t aggregate your data?
Of course not! Since everyone, including manufacturers, will rely on the practice of inference. Aggregation is in fact necessary for interoperable trade in the drug supply chain.
What most manufacturers and distributors don’t yet realize is that aggregated information will become a key component of their exception handling strategy and their ability to demonstrate control.
When you talk to most serialization and traceability professionals about aggregation they will always speak about “parent child relationships” of the packaging levels. Once in a while someone will point out that all of the serialized packaging levels are a child of both the product itself (NDC) and a specific Lot which produced the packaged products (GTINs). In fact, GTINs are children of the NDC - the parent.
A company's ability to demonstrate operational control and manage exceptions can be built upon this simple fact.
Trust.med
Trust.med has established an interactive approach to complement your current solutions and/or SOPs using the .med secure domain to interoperably connect with the market.
We can solve your DSCSA compliance gaps for data exchange with our ability to establish secure, trusted, and dedicated replication endpoints for the purposes of drop ship, exchanging enhanced recall data, and sending transaction information. The .med secure domain provides your company a powerful discovery service with the GS1 conformant set of Digital Link internet resolvers.
These GS1 based .med domains easily interface with your existing solutions. The Trust.med resolver service puts you in control. Every .med domain is a product specific resolver with authoritative information access points controlled by your company.
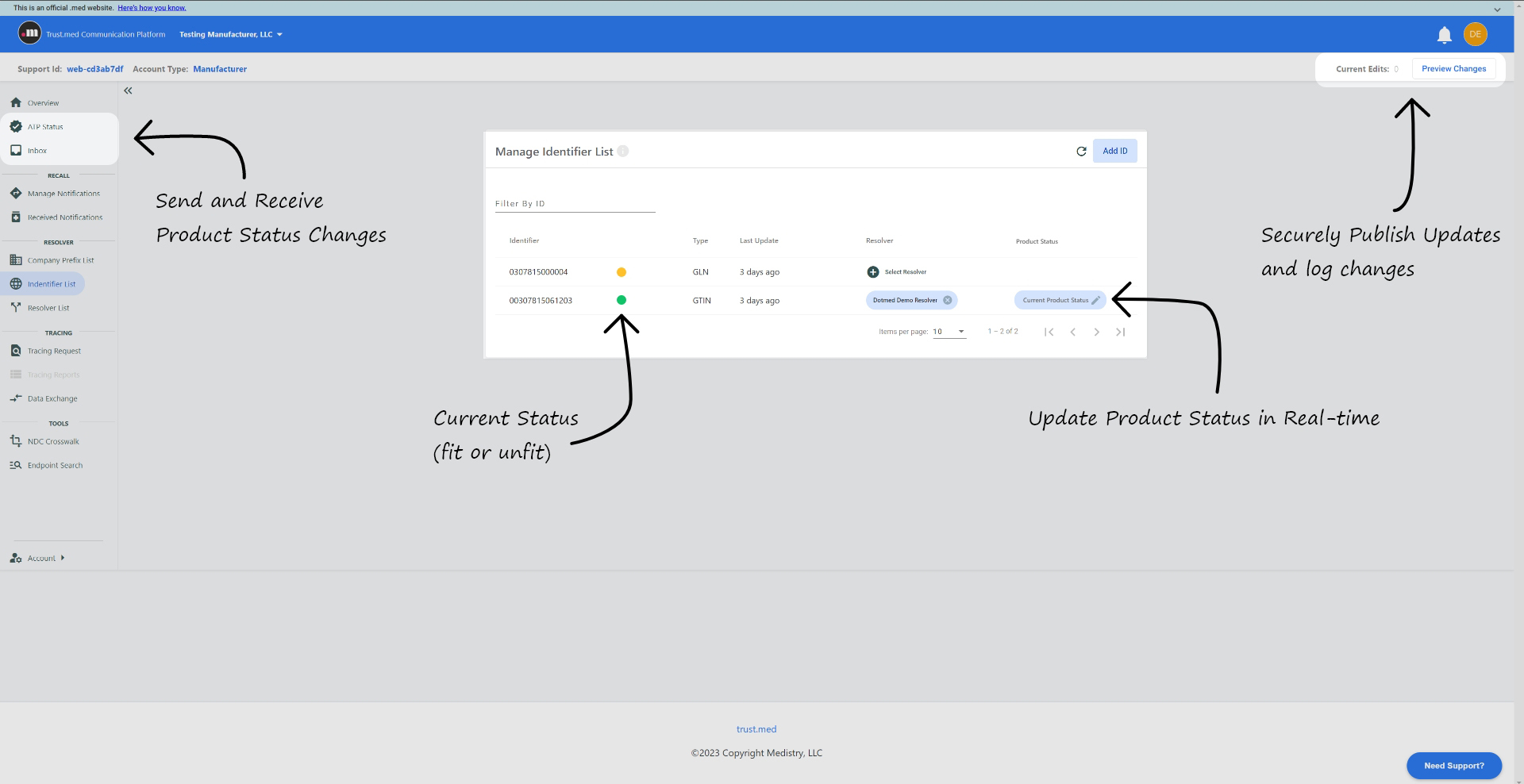
Additionally, where solution gaps exist, we offer secure and dedicated workspaces for the management of external communications. Manage requests such as product Trace and Transaction Exceptions while providing needed segmentation from your enterprise systems. Systems that typically cannot be enabled to directly respond. Your users control all activities as relevant data is accumulated and organized according to your requirements.
To further assure DSCSA compliance, Trust.Med compliments the ability of operating within a secure domain by offering a compliant ATP solution.
Contact me to learn more about Trust.med or to talk about aggregation and hear my perspective on managing those exceptions in a controlled manner.

Lloyd Mager
Market Solutions Officer
Subscribe for updates
Learn more



